Knocked on wood
C the UNSEEN" doesn't just apply to Chemnitz - other supposedly well-known areas can also suddenly come up with surprises. For example, the geology of the region around Meißen was considered well researched until 2016, when a citizen scientist discovered the first silicified wood in the Meißen area. The find from a kaolin mine southwest of Meißen prompted an excavation, during the course of which several trunks, some of which were several meters long, came to light.
The preservation of the timbers revealed only a few anatomical details and did not allow any more precise determination than the form genus Agathoxylon. Stems of this type belong to gymnosperms (gymnosperms), like today's conifers and ginkgos. However, they also occurred in the Paleozoic era (Palaeozoic era), making it impossible to determine their age from the fossils themselves. However, the positional relationships indicate that the trees were embedded before the onset of volcanism at the end of the Carboniferous period and are therefore older than 300 million years.
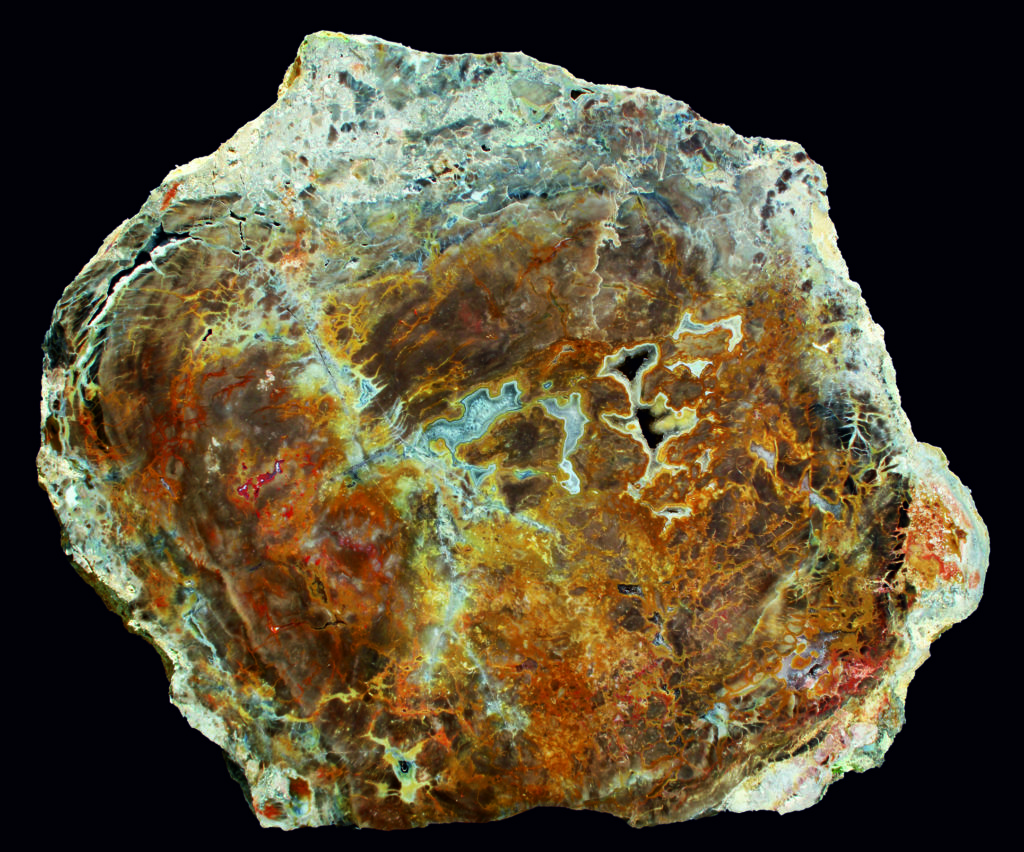
The color variation of the wood from golden yellow to blue-grey is striking. Under UV light, they show a division into a core and a peripheral zone, which provides insights into the fossilization process. The uniform luminescence suggests that all the trees must have been silicified in a single event. The substance that encloses them today is kaolin - a later weathering product that is mined for porcelain production.
Many discoveries are thanks to the tireless efforts of citizen scientists. Siliceous woods fascinate with their splendid colors - and for scientists they are archives of life in past geological eras. The Museum für Naturkunde Chemnitz enjoys worldwide recognition as a location for research and exhibitions on fossil forests.